environmental SCIENTIST | Unearthing global megatrends in land condition | September 2023
Paul Nathanail and Shaun Grey explain the difficulties of remediating sites affected by these complex chemicals.
Introduction
The European Environment Agency identified 11 global megatrends that will affect ecological and societal resilience and which have significant consequences for Europe.1 One such megatrend, Increasing Environmental Pollution (GMT10), has been recognised as a global transboundary problem that affects air, water, soil and whole ecosystems. Three main human activities are linked to this pollution: fossil fuel combustion, the application of synthetic fertilisers, and the growing use and complexity of chemicals.
It is an increasing challenge to assess and manage the risk presented by a group of complex chemicals when released into the environment, and it poses a land contamination issue. Growing concerns over the level of risk of per- and polyfluorinated alkyl substances (PFAS) to human health and the environment have resulted in considerable regulatory, scientific and engineering developments. They have even been the subject of the 2019 Hollywood film Dark Waters.
|
|
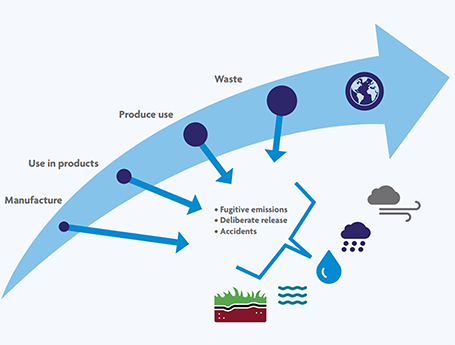
| Figure 1. The PFAS life cycle. All PFAS were manufactured for use in products. During their use and upon disposal, many PFAS are released into the environment, and they have been found in some of the remotest parts of the planet. (© Land Quality Management) |
What are PFAS?
PFAS are a group of synthetic chemicals made over the past 80 years to serve many purposes. The basic building block of a PFAS is a chain of carbons – the alkyl part of the name. As a tetravalent atom, carbon needs to form four covalent bonds. In aliphatic hydrocarbons, a carbon does this by sharing electrons with another carbon or two and with hydrogens. In a PFAS some or all the hydrogens are replaced by fluorine – the fluoro part of the name. If all the hydrogen atoms bonded to a carbon are replaced by a fluorine atom, then the substance is a perfluoroalkyl substance – the same per- that we find in the chlorinated solvent named perchloroethene (PCE). If all the hydrogens on at least one – although not all – the carbons in the chain have been substituted with fluorines, then we have a polyfluorinated alkyl substance.
There are thousands of PFAS! The PFAS universe can be broken down into galaxies, many with different functional groups attached to one end of the carbon chain or with other atoms such as oxygen or sulphur acting as a bridge between two or more chains of carbons. Within each galaxy there will be substances with different numbers of carbons in the chain – from one (C1) to six (C6), eight (C8) and more… many more.
In an attempt to promote a consistent approach to the regulation and management of these chemicals, the Organisation for Economic Co-operation and Development (OECD) has provided a definition of PFAS (see Box 1).2 However, the recent joint Health and Safety Executive Risk Management Options Analysis (RMOA) for PFAS has adopted a narrower definition.3
|
Box 1: THE OECD DEFINITION OF PFAS ‘Any fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/Ia atom attached to it), i.e. with a few noted exceptions, any chemical with at least a perfluorinated methyl group (-CF3) or a perfluorinated methylene group (-CF2-) is a PFAS'.2 Note: a H = hydrogen; Cl = chorine; Br = bromine; I = iodine |
PFAS have been used in many applications. They remain in use today and new and novel forms are still being developed, albeit within an increasingly regulated market. Due to their excellent water, soil, stain and heat resistance, PFAS have been used in biocides, flame retardants, firefighting foams, construction materials, floor polishes, protective clothing, food packaging, medical devices and electrical wires.
Where have PFAS been used and found?
PFAS have been detected worldwide and can be found on sites far away from point sources. Ambient levels in soil and concentrations in rainwater can exceed relevant screening levels.4 However they were manufactured, stored and used, understanding a site’s history is an important part of a preliminary risk assessment if PFAS land contamination is to be adequately considered.
Industrial sites with a history of using PFAS to fight certain types of fire, or to make clothing waterproof, food wrapping grease-proof, furniture fire resistant, and many more uses should require an intrusive investigation. Fighting fires of flammable liquids (Class B fires) has historically involved using PFAS-based aqueous film-forming foams (AFFF). Training to fight such fires and washing down appliances after a firefighting incident are likely to have resulted in AFFF PFAS getting into the ground – through soil, water courses and storm drains. The widespread use of PFAS in firefighting foams has seen pollution identified on civilian and military airfields and training areas. Fuel-storage sites have also been impacted during firefighting incidents and training activities, and from potential foam storage spills.5
In England, Environment Agency sampling programmes have identified PFAS in surface and ground water and in estuarine sediments.6 Limited sampling of mammals and fish has also confirmed the presence of PFAS. Leachates from landfills where PFAS-containing wastes were disposed of – especially the more soluble ones – are likely to be contaminated and they are also found in discharge from wastewater treatment plants and on agricultural land where sewerage sludge has been spread.
PFAS have been so widely used that they may be present in most media (soil, sediment, waste, wood, tarmac, bricks, concrete, glass, coatings and carpets).
How do PFAS behave?
PFAS are very stable and resistant to chemical attack – largely because of the strength of the carbon–fluorine bonds. They can withstand high temperatures and resist chemical and biological degradation.
The PFAS of main environmental concern are those that are toxic, soluble, persistent and bioaccumulative – mainly the long-chain perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkane sulphonic acids (PFSAs). Perfluorooctane sulphonic acid (PFOS) is the best-known PFSA (used in firefighting foams) and perfluorooctanoic acid (PFOA) is the best-known PFCA (used in waterproofing fabric membranes) – but others are also common and hazardous. The structure of the most problematic PFAS gives them peculiar behaviours. Specifically, since the end of the carbon chain with a functional group is hydrophilic (attracting water) and the other end is hydrophobic (repelling water), they behave like surfactants – akin to washing-up detergents. This allows PFAS to foam and resist water and grease – the properties that make them useful.
PFAS behaviour depends on chain length. PFOA and PFOS are long-chain PFAS whose characteristics make them good surfactants, resulting in them accumulating at soil, water and air interfaces. Long-chain PFAS have also been observed on the surface of concrete at concentrations high enough to act as decades-long sources of pollution and potentially preventing the reuse of crushed concrete. Short-chain PFAS are more soluble and have a lower potential to bioaccumulate relative to long-chain PFAS.
Most PFAS do not readily partition to air and have a low to moderate sorption to soil. This, coupled with high solubility, results in groundwater plumes that can reach multiple kilometres – longer than either hydrocarbon or chlorinated solvent plumes.4 Soil mineralogy and groundwater geochemistry, together with the individual type of PFAS, control mobility – for example, solubility decreases in brackish and saline conditions. Therefore, detailed, site-specific and chemical knowledge and understanding are required to comprehend and model PFAS mobility.
Disposal and risks
PFAS have been deposited into the environment through a multitude of waste activities, including controlled and uncontrolled discharge to water, incineration and open burning, landfilling, uncontrolled tipping and even littering. PFAS are very mobile when released into the environment and have been found in rivers, in lakes and in the human body. Many bioaccumulate and have been associated with adverse effects to human health and wildlife following exposure events.
Risks to human health. The toxicity of most PFAS remains poorly understood. However, those that have been relatively well studied have been associated with adverse effects on the liver, immune system and reproduction and are potentially carcinogenic in humans and animals. Unlike lipo-philic hydrocarbon or chlorinated solvent molecules, PFAS are protein-philic. Short-chain PFAS are also more likely to be excreted than long-chain PFAS. PFOS and PFOA can cross the placenta in humans and animals, and breast milk may transfer PFAS from mother to child.7
Risks to ecosystems. The ecotoxicity of PFAS varies across species. Most studies looked at exposure to PFOS and PFOA in only a few species (e.g. zebrafish, earthworms and chickens) and determined responses in growth, reproduction and mortality as well as biochemical effects and gene expression. PFOS specifically has been shown to increase bee mortality and affect colonies. UK ecological soil-screening levels are based on broad assumptions about secondary poisoning of birds through the food chain from PFOS and PFOA in soil that is consumed by earthworms. Studies of the effects of exposure to PFAS on survival, growth and reproduction indicate some level of toxicity, but the datasets are small and mainly for avian and mammalian wildlife species where other pollutants and stressors may prevent definitive associations of adverse effects with PFAS exposure.4
How can PFAS be remediated in soil?
Remediation involves managing unacceptable risk by demonstrably breaking the source–pathway–receptor linkage. The different chemical structures, including carbon chain length and functional group, mean that not all PFAS are amenable to the same remediation approaches. The persistence of the more problematic PFAS means in situ destruction is not yet a practical solution. Immobilisation and stabilisation can be achieved by mixing a sorbing agent, such as an activated clay.
Remediation of PFAS in soil involves in situ stabilisation or excavation, extraction and destruction. Several ex situ methods have also been applied. Excavation and disposal to landfill of PFAS-contaminated soils is only possible if levels do not exceed Stockholm Convention waste limits. Alternatively, high-temperature incineration (>1,100C) of soil can be used to destroy PFAS, but this requires very good process control so is expensive and costs may be prohibitive.
Soil washing may be considered to concentrate the PFAS if the soil has relatively low silt, clay and organic content (i.e. fines). The treatment and disposal of extracted water and fines may be complex and expensive. Various containment methods have also been used, such as capping soils in situ and engineering stockpiles to prevent infiltration and leaching of PFAS. Liability and continued management remain with the site owner, while restrictions may be placed on redevelopment of a site.
How can PFAS be remediated in groundwater?
Groundwater treatments revolve around immobilising PFAS in the ground or abstracting water and treating it. Immobilising agents can be injected that capture and remove PFAS. Above-ground treatments either use filters to remove PFAS or blast air through the water to create a PFAS-rich foam that is separated and dispatched for PFAS destruction. Different filter media are needed for short- and long-chain PFAS molecules. Treatment of PFAS-contaminated groundwater using abstraction and granular activated carbon to remove long-chain PFAS and ion exchange columns for short-chain PFAS are proven technologies, but life cycle and maintenance costs are high.7
Other techniques, such as reverse osmosis and nanofiltration, have been demonstrated to be effective. These methods are expensive and presently only suitable for large-scale drinking water treatment. Each method separates the PFAS, in some cases on to a solid medium (e.g. granular activated carbon). Both PFAS and the waste media require a suitable destruction, recovery or disposal route.
Guidance and competencies
Across the UK, regulators point those responsible for managing the risks from land contamination to the generic Land Contamination Risk Management (LCRM) guidance.8 LCRM adopts a three-stage framework of risk assessment, remediation options appraisal, and remediation implementation and verification. It sets out the standards expected in the investigation, sampling and assessment of soil and water contamination. There is also coverage of what constitutes a competent person to undertake assessment and management, and they are expected to have appropriate knowledge, skills, experience and qualifications in the specific area of work and, importantly, in the type of contamination being managed. Guidance due out later in 2023 from CIRIA will explore the PFAS-specific aspects of complying with LCRM.
The investigation, assessment and remediation of PFAS needs a multi-disciplinary team – not least because of the rapid developments in our understanding of the fate, transport and toxicity of PFAS and of the technologies available to analyse samples and to treat soil and water. Specialists in Land Condition (SiLC) are well placed to bring together and marshal the necessary competencies in soil, groundwater, geochemistry, toxicology and process engineering. These are chartered professionals – for example, through the IES – who have then gone on to demonstrate the broader skills and knowledge needed to manage land condition.9
Current initiatives
The Environment Agency has an ongoing programme of work to understand the scale and nature of PFAS contamination. Phase 4 is currently underway with projects designed to understand ambient levels of PFAS in soil, develop internal guidance for regulators, explore how applying Part IIA of the Environmental Protection Act 1990 (as amended) works in practice for PFAS, and to characterise PFAS in landfill gas and leachates.
The RMOA for PFAS under UK REACH (which regulates chemical substances on the British market) examined information on the intrinsic hazards, uses and routes of exposure to PFAS. However, the RMOA excludes risks arising from historic or discontinued uses of PFAS.2
CIRIA is preparing technical guidance on the investigation, assessment and remediation of the short- and long-chain PFAS encountered most often in soil and groundwater. As well as the investigation, assessment and remediation of PFAS in soil and water, it also describes the range of targeted and non-targeted analytical techniques used to quantify PFAS.4
At a European level, both the EU Common Forum of policy-makers and regulators and the NICOLE network of industry, service providers and academia have working groups on PFAS seeking to create a shared understanding of the regulatory, analytical, remediation and risk assessment aspects of PFAS contamination. Australia has been at the forefront of efforts to deal with PFAS contamination in groundwater, at least in part because of high-profile pollution of drinking water supplies around defence sites. The USA has several large and well-funded research programmes. Efforts to reduce the potential magnitude of the US Department of Defense’s PFAS liability have driven research on AFFF-impacted sites since 2011 to evaluate a range of potential treatment technologies, ecotoxicological effects and improved AFFF site characterisation. More recently the USA’s Bipartisan Infrastructure Law has provided substantial funding to assess and tackle PFAS contamination – especially that affecting disadvantaged communities.10
Conclusion
Managing the risks this complex universe of chemicals poses in the environment is clearly a challenging conundrum. It requires a multi-disciplinary approach to maintain awareness of the developing science and to apply it in remediating the adverse effects. Such activities are a prime consideration under GMT10. Chemicals such as PFAS are also a factor intimately involved with other global megatrends, contributing to degrading and adding pressure on ecosystems (GMT8) and requiring transboundary management that demands a coherent international governance against a background of diversifying approaches (GMT11).1 In the context of managing land contamination risks, the multi-disciplinary expertise of land contamination specialists is likely to become even more important.
Paul Nathanail, CGeol, SiLC, SQP chairs the Specialist in Land Condition Register board of directors having previously served as the Geological Society Technical Representative on the SiLC Professional and Technical Panel. He is a director of Land Quality Management (LQM). Paul is currently drafting technical guidance, including for CIRIA, on managing PFAS in soil and water. He also co-chairs the NICOLE PFAS working group.
Shaun Grey, MIEMA, CEnv, SiLC, is the IEMA Technical Representative on the SiLC Professional and Technical Panel. His experience in land contamination management comes from contracting, consulting and local authority regulation. Shaun joined the UK Ministry of Defence in 2004 and has worked across the organisation in environmental and safety management roles.
References
- European Environment Agency (2015) Global megatrends. https://www.eea.europa.eu/soer/2015/global (Accessed: 23 May 2023).
- Organisation for Economic Co-Operation and Development (2021) Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances: Recommendations and Practical Guidance. Series on Risk Management, No. 61. https://one.oecd.org/document/ENV/CBC/MONO(2021)25/En/pdf (Accessed: 23 May 2023).
- Health and Safety Executive (2023) Regulatory management option analysis (RMOA). https://www.hse.gov.uk/reach/rmoa.htm (Accessed: 23 May 2023).
- Nathanail, C.P., Ross, I., Williams, G. and Nathanail, J. (2023) Good Practice Guidance: Some Per- and Polyfluorinated Alkyl Substances (PFAS) in Soil and the Water Environment. In draft. London: CIRIA.
- CL:AIRE (2023) An Overview of the Uses of PFAS to Assist with Identification of Sites of Concern. Technical Bulletin TB 22. https://www.claire.co.uk/component/phocadownload/category/17-technical-b... (Accessed: 23 May 2023).
- Environment Agency (2021) Poly- and Per-fluoroalkyl Substances (PFAS): Sources, Pathways and Environmental Data. Chief Scientist’s Group Report. https://assets.publishing.service.gov.uk/government/uploads/system/uploa... (Accessed: 23 May 2023).
- CL:AIRE (2019) Managing Risks and Liabilities associated with Per- and Poly-fluoroalkyl Substances (PFASs). Technical Bulletin TB 19. https://www.claire.co.uk/tb-19-managing-risks-and-liabilities-associated... (Accessed: 23 May 2023).
- Environment Agency (2021) Guidance: Land contamination risk management (LCRM). https://www.gov.uk/government/publications/land-contamination-risk-manag... (Accessed: 23 May 2023).
- Specialist in Land Condition (no date) SiLC register. https://www.silc.org.uk/ (Accessed: 23 May 2023).
- White House (2023) Fact sheet: Biden–Harris Administration takes new action to protect communities from PFAS pollution. https://www.whitehouse.gov/briefing-room/statements-releases/2023/03/14/... (Accessed: 23 May 2023).